Strategies to ensure credibility and transparency
In addition to the practices defined above, there are some standard strategies that all research laboratories should follow to enhance credibility and transparency during clinical trials. In terms of scientific rigour and documentation, the use of standardised scientific protocols and methodologies, such as randomised blinded evaluations or placebo-controlled studies, adds credibility and validity to trial results. At the same time, keeping detailed records describing trial procedures, participant demographics, and observed changes is essential.
Independent verification and peer review can also be a good strategy to increase the relevance of the results. Three different levels of verification can be established: first, the results are obtained after internal 'informal' studies, without an external CRO being involved in the measurements and analysis of the results. Second, the CRO is responsible for the clinical trial and the full final report includes all raw data, analysis of results and conclusions made by an experienced professional. Third, the results obtained in the final report provided by the CRO are published in a scientific journal and reviewed by one or more experts with similar competencies. It is widely recognised that the reliability of 'in-house' results has to be lower than when the results are obtained and/or reviewed by external parties such as CROs or scientific journals.
Another important marketing-related strategy is to emphasise realistic expectations. Educating consumers about realistic results is crucial. Using subjective assessments to claim high percentages of improvement without having objective instrumental validation of those results could be bread for today and hunger for tomorrow, as the market is more knowledgeable today than it was 20 years ago and will soon understand that cosmetic topical applications cannot result in the same 'percentages' as clinical surgeries. Avoiding extravagant promises or unrealistic transformations in advertising or promotional material is essential. In addition, emphasising that individual responses may vary according to various factors helps to manage expectations.
Statistical analysis in the evaluation of cosmetic products
Rigorous statistical analysis is an indispensable factor in the field of cosmetic clinical trials, acting as the cornerstone for reliable and reproducible conclusions. In efficacy and safety assessment, the use of robust statistical methodologies is imperative to distil meaningful insights from complex data sets. Statistical analysis not only helps to decipher the subtleties of skin care outcomes, but also ensures the precision and accuracy of experimental results. By employing statistical rigour, clinical researchers can effectively quantify the impact of interventions, discern trends amidst variability, and determine the significance of observed effects, thereby strengthening the credibility and reliability of findings. This meticulous approach not only elevates the quality of evidence, but also fosters consistency and replicability in results, thereby strengthening confidence in the conclusions drawn from clinical evidence.
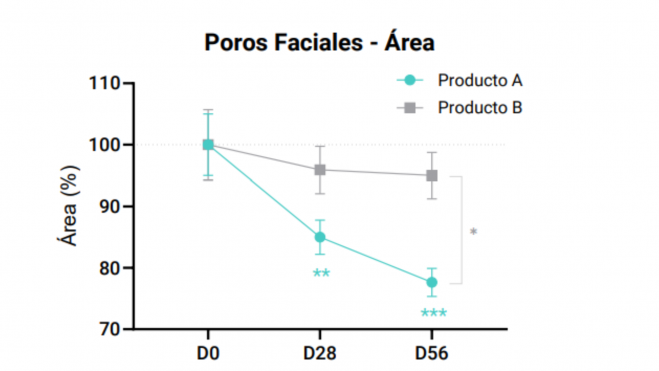
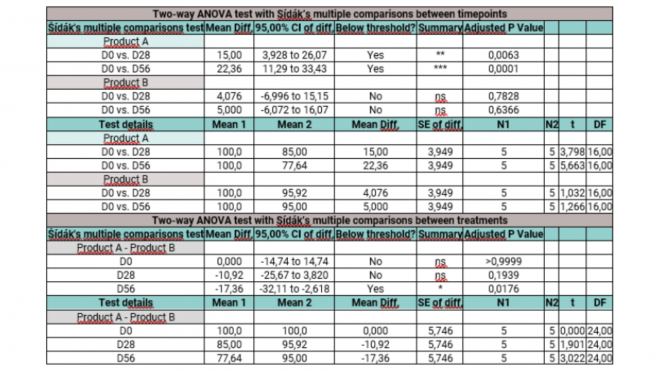
The following appearances have to consider for a rigorous statistical analysis and reliable in the clinical studies with ingredients or cosmetic products: or Compilation of data and measurement:
- Data collection and measurement: Establishing quantitative metrics to assess changes in skin characteristics, such as wrinkle depth, skin hydration levels or pigmentation, is crucial. Using standardised instrumental measurement tools, such as spectrophotometry or image analysis software, rather than subjective clinical observations, ensures consistency in data collection.
- Descriptive statistics: Using descriptive statistics, such as mean, median and standard deviation, summarises the central tendency and variability of data collected from participants before and after product use, considering the inherent intravariability of human clinical trials. Presenting graphical representations, such as box plots or histograms, can also be useful to visually illustrate the distribution of observed changes among participants. In marketing journals, it is common to see graphs of results without variances or error bars, which can lead to misinterpretation of the effects obtained.
-
Paired Student's t-tests or Analysis of Variance (ANOVA): The choice of method, whether paired Student's t-test, ordinary one-way ANOVA or two-way ANOVA, depends on the complexity of the experimental design, the number of variables involved and the specific research questions addressed within the clinical trials. The paired Student's t-test is appropriate when examining the effects of a single intervention on a single group, making comparisons between two related data sets. It is beneficial for before and after assessments within the same group, such as before and after treatment measurements in the same subjects. Using the paired Student's t-test for all types of analyses are common errors that lead to misinterpretations and misleading conclusions. On the other hand, one-way ANOVA is the rigorous method to apply when dealing with an independent variable that has more than two levels, allowing comparison of means between different groups or treatments. This method is suitable for scenarios with more than two data sets and should be applied in studies with one treatment and more than two time points. Finally, two-way ANOVA is ideal when there are two independent variables affecting the outcome, so that we can assess how these variables simultaneously influence the dependent variable, as well as their interaction This statistical tool is best suited for designs with multiple treatments in different groups and additional factors or variables affecting the outcome. This should be applied in placebo-controlled studies.
Translated with DeepL.com (free version)
- Confidence intervals and p-values: Calculating confidence intervals around mean differences provides a range within which the true product effect is likely to lie. Interpreting p-values derived from statistical tests helps to determine the significance of observed changes. A p-value less than 0.05 indicates statistically significant results. Presenting statistically significant findings along with their clinical relevance (*, **, ***, or ****) is essential for a correct interpretation of the results. Discussing the practical implications helps to understand the significance of the observed changes. Graphical plots of results without confidence intervals and error bars are often observed, which can lead to erroneous interpretations. In addition, expressing confidence or uncertainty in conclusions derived from statistical analyses, based on the strength of the evidence and statistical rigor, adds credibility to the findings.
- Calculation of the population size: The number of volunteers included in a clinical trial is an essential aspect to consider, since it is clear that the results obtained for a small group of 5 volunteers cannot be as reliable and realistic as for a large group of 100 volunteers. The number of volunteers recruited for the study must be sufficient to determine statistically significant differences; otherwise, the low power of the statistical analysis could jeopardize the conclusions of the evaluation. According to different mechanisms to determine the size of a population, it has been widely recognized in cosmetic clinical trials that at least 20 volunteers are always necessary to be able to carry out a study with the minimum standards of reproducibility; a panel of between 30 and 40 people per group being considered the ideal size in relation to the quality of the results obtained/investment made for the development of the study. Recognizing limitations, such as small sample sizes, is crucial to correctly interpret the presence or absence of statistical findings.
- Controlling for covariates: Controlling for potential confounding variables, such as age, gender, as well as baseline skin conditions, using techniques such as multivariate analysis or regression analysis, could help isolate the true effect of the product. However, this type of analysis of controls and additional analysis is more typical of the pharmaceutical industry than of the cosmetic industry.
- Handling missing data and outliers: Addressing missing data through appropriate imputation techniques ensures the robustness of the analysis. Removing outliers or employing robust statistical methods avoids undue influence on the results. Different methods, such as ROUT (robust regression and outlier removal), can be used to identify outliers in the raw data, with a Q coefficient determined by how aggressively the method defines the outlier (from 0.1% for removing definite outliers to 10% for removing probable outliers). To maintain the human intravariability inherent in clinical testing with volunteers, it may be of interest to use high levels of constraint (Q).
Discussion and conclusion
Comparative clinical studies are an indispensable tool for evaluating the efficacy of cosmetic ingredients and products. Today, the data obtained from clinical studies are one of the fundamental aspects for the future of product development. However, their reliability and integrity depend on strict adherence to standardized practices and ethical considerations. Transparency, scientific rigor and management of consumer expectations are critical to presenting authentic representations of product outcomes. Recognizing limitations and potential biases inherent in visual representations underscores the need for caution when interpreting and presenting before-and-after images. Balancing the display of transformative effects with the scrutiny of rigorous evaluation is crucial to maintaining credibility in cosmetic product evaluation.
By maintaining best clinical practices and ethical integrity, such as consistency in experimental conditions, encouraging participants to adopt similar postures and facial expressions at different measurement times, using high-resolution and unaltered images, establishing clear timelines and protocols for treatment delivery, documenting procedures, ensuring participant compliance, using reliable innovative instrumental technologies and not solely subjective assessments, limiting the impact of external factors, avoiding ethical issues with corresponding informed consents, employing sound statistical methods, and transparently reporting procedures and procedures in a transparent manner, use reliable innovative instrumental technologies and not just subjective assessments, limit the impact of external factors, avoid ethical issues with appropriate informed consents, employ sound statistical methods, and transparently report procedures and results of analysis, cosmetic product evaluations can greatly improve their scientific validity. Statistical analysis is essential to complement visual representations in comparative tests, providing quantitative evidence of product efficacy and strengthening the credibility of claims about the effects of cosmetic products on skin health and appearance. Together, the cosmetics industry can build trust, improve accountability and empower consumers to make informed decisions by applying sound and rigorous practices.
On the other hand, establishing common criteria for validating claims among different laboratories and research organizations is crucial to ensure fair competition within the industry. Consistent standards serve as a cornerstone for reliability and credibility, enabling proper and reproducible evaluation and comparison of products. When all parties adhere to the same rules, it fosters transparency, builds consumer confidence and mitigates misleading or exaggerated claims. In addition, standardized criteria simplify the evaluation process, allowing companies to invest resources more effectively in research and development, ultimately leading to the creation of safer and more effective cosmetic products. This harmonization benefits not only the industry but also empowers consumers by providing them with accurate and consistent information to make informed decisions about the products they use on their bodies.


